SOLVED QUESTION 4 Given the following reaction CaO(s) + H2O() Ca(OH)2(s) AH =64.8 kJ how many

CaO(s) + H2O(l) Ca(OH)2(aq) Chemistry. 1 Answer Dwight Jan 28, 2018 The heat of reaction is #DeltaH = -95.3 (kJ. (g°C) xx 7.3°C = 1529.35 J# or 1.53 kJ (rounded to three digits). Since this was due to 1.045g of CaO, which has a molar mass of 56.1 g, the number of moles of CaO used was #1.045g/56.1 g/"mol" = 0.01605 "mol"# Therefore, the.
How to balance HNO3+ Ca(OH)2 Ca(NO3)2 + H2O YouTube

but some claim that since CaO is an weak base, it should be combined with OH, thus CaO(aq) + H2O(aq) -> Ca(OH)2(aq) Which one is right and why? Here's the best way to solve it. Who are the experts? Experts have been vetted by Chegg as specialists in this subject. Expert-verified.
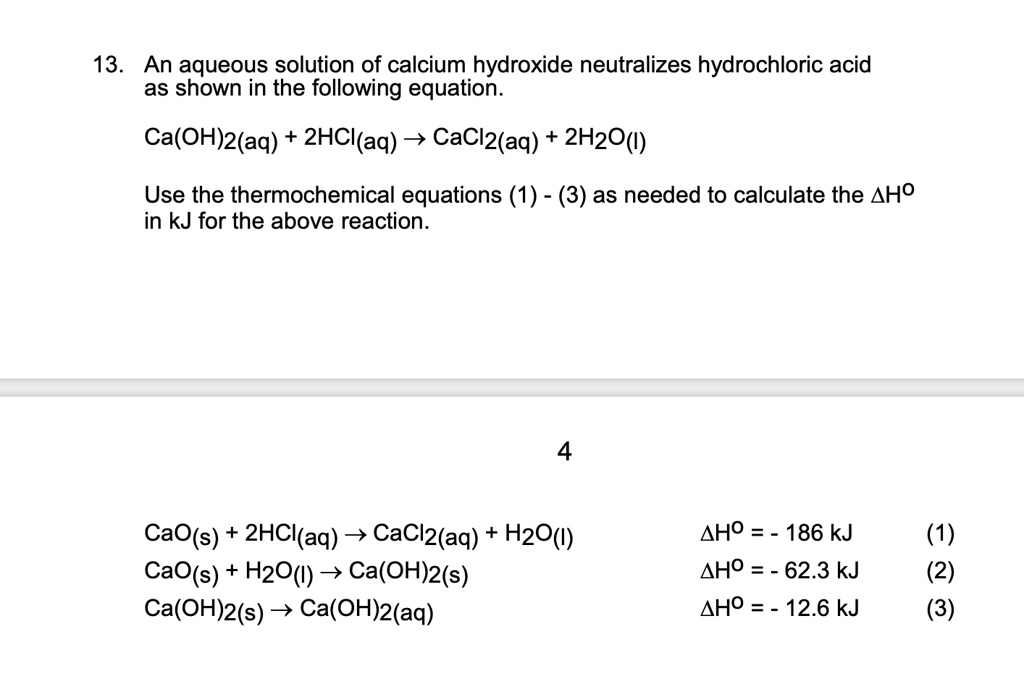
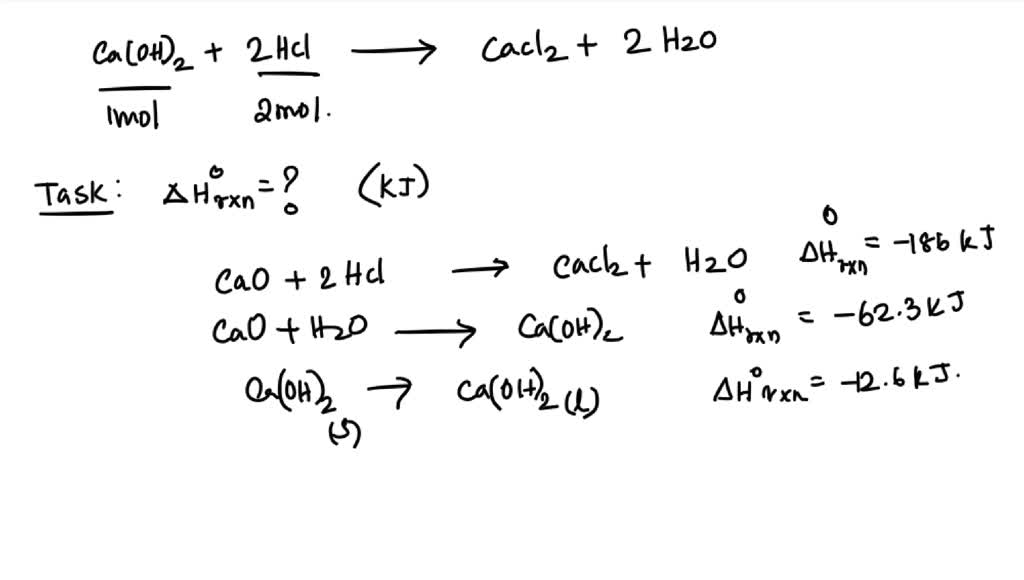
SOLVED 13 An aqueous solution of calcium hydroxide neutralizes hydrochloric acid as shown in
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of Ca + 2H2O = Ca (OH)2 + H2, the equation is balanced.
Type of Reaction for CaO + H2O = Ca(OH)2 YouTube

How to Balance: CaO + H 2 O → Ca (OH) 2. Word equation: Calcium oxide plus Water → Calcium hydroxide. Type of Chemical Reaction: For this reaction we have a combination reaction. Balancing Strategies: This is an exothermic chemical reaction and gives off heat. Hint-1. Hint-2. Show Balanced Equation.
How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O (Calcium Carbonate + Hydrochloric Acid) YouTube

Calcium Oxide + Water = Calcium Hydroxide. CaO + H2O = Ca (OH)2 is a Synthesis reaction where one mole of Calcium Oxide [CaO] and one mole of Water [H 2 O] combine to form one mole of Calcium Hydroxide [Ca (OH) 2] Show Chemical Structure Image. For a small piece of calcium oxide into a test tube, a few drops of water to calcium oxide.
Balance the following equations i) CaCO3 + HCl → CaCl2 + CO2 ↑ + H2O ii) Na + H2O → NaOH + H2 ↑

Identify the oxidation number of hydrogen in each reactant and product for the first reaction, SO2 (g) + H2O (l) → H2SO3 (aq), assuming the oxidation number of hydrogen in H2O as +1 and oxygen as -2.
how to balance caco3 hcl cacl2 co2 h2o YouTube

The reaction is very favorite energetically so you don't have the equilibrium you mentioned: when Ca(OH)2 is formed you will not have CaO back until you provide sufficient heat and temperature. The second reaction is right: Ca OH 2 s Ca aq 2OH aq. The solubility of Ca(OH)2 is 0.173 g/100 mL (20 °C).
thí nghiệm CaO tác dụng với H2O tạo thành Ca(OH)2 làm quỳ tím hóa xanh YouTube

CaO(s) + H 2 O(l) → Ca(OH) 2 (aq) + Heat. This reaction can be classified as (A) Combination reaction (B) Exothermic reaction (C) Endothermic reaction (D) Oxidation reaction Which of the following is a correct option? View Solution. Q3. Calcium oxide reacts vigorously with water to produce slaked lime.
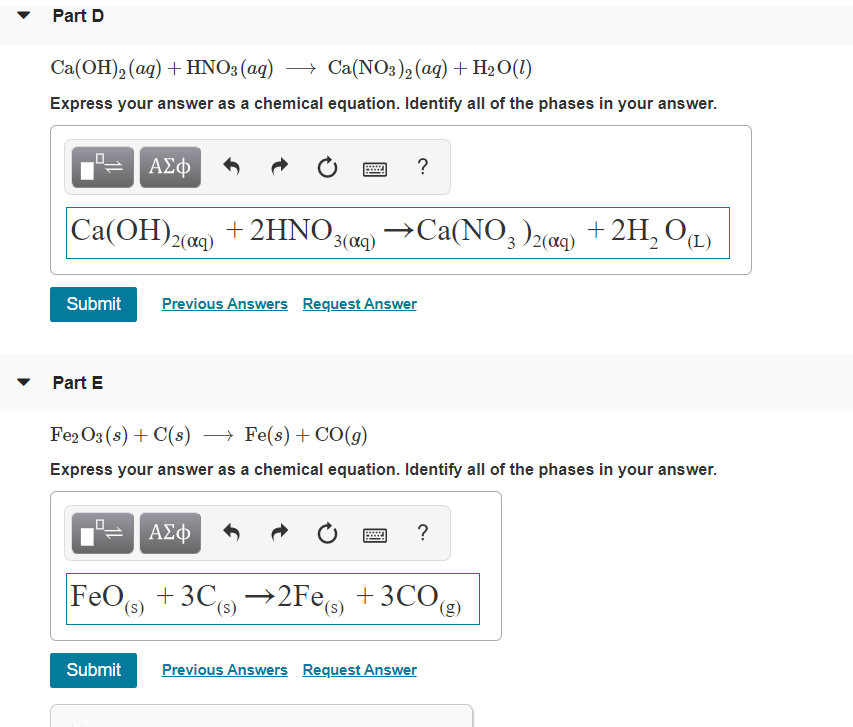
Solved Part D Ca(OH)2(aq) + HNO3(aq) + Ca(NO3)2(aq) + H2O(1)
The reaction between calcium oxide and water produces aqueous calcium hydroxide CaO(s) + H2O(l) ---> Ca(OH)2(aq) In the reaction of 16.7 g CaO with 10.7 g H2O, assuming CaO is limiting, how much excess H2O (in grams) is leftover at the end of the reaction? Enter your answer as a number only 2. 5.000 g of a compound containing C, H and O was
How to Balance CaO + H2O = Ca(OH)2 (Calcium oxide plus Water) YouTube

In the given reaction. CaO(s) + H 2 O(l) → Ca(OH) 2 (aq). Calcium oxide Water Calcium hydroxide. Part i) : lime (CaO) combines with water to form slaked lime Ca(OH) 2 (aq). Hence two substances are combined to form a single substance, therefore it is a combination reaction.
How to balance HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O YouTube

The formation of slaked lime (calcium hydroxide, Ca(OH) 2) when water is added to lime (CaO) is exothermic. CaO(s) + H2O (l) → Ca(OH) 2 (s) This reaction occurs when water is added to dry portland cement to make concrete, and heat evolution of energy as heat is evident because the mixture becomes warm. Not all reactions are exothermic (or.
When NH4Cl is treated with CaO we get CaCl2, Ca(OH)2 and X as its products. Where X is

There are three main steps for writing the net ionic equation for CaO + H2O = Ca(OH)2 (Calcium oxide + Water). First, we balance the molecular equation. Seco.
(i) CaO(s) + H2O (l) = Ca (OH)2(s) , Δ H180^oC = 15.26 kcal (ii) H2O(l) = H2(g) + 12 O2 (g

(aq) represents solution in water. Cao (s) + H 2 O (l) → C a (O H) 2 (aq) + heat. From the above equation, the following observations can be made. (a) CaO is solid, (b) CaO and H 2 O reacts to produce product, So CaO is reactive with water. (c) H 2 O is in liquid state and not vapour. (d) C a (O H) 2 forms a solution with water
SOLVED Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide

Study with Quizlet and memorize flashcards containing terms like Given that CaO(s) + H2O(l) -> Ca(OH)2(s), (Delta) DH°rxn = -64.8 kJ/mol, how many grams of CaO must react in order to liberate 525 kJ of heat?, A 100. mL sample of 0.200 M aqueous hydrochloric acid is added to 100. mL of 0.200 M aqueous ammonia in a calorimeter whose heat capacity (excluding any water) is 480.
Neutralization reactions ppt download
+%2B+H2O+(l)+%EF%83%A0+Ca(OH)2+(aq).jpg)
Classify the Following Reaction into Different Type: Cao(S) + H2o(L) → Ca(Oh)2(Aq) CBSE English Medium Class 10. Question Papers 998. Textbook Solutions 34075. MCQ Online Mock Tests 19. Important Solutions 5804. Concept Notes & Videos 213. Time Tables 15. Syllabus.
SOLVED 13 An aqueous solution of calcium hydroxide neutralizes hydrochloric acid as shown in
In this video we determine the type of chemical reaction for the equation CaO + H2O = Ca(OH)2 (Calcium oxide + Water).Since we have a two substances combinin.